

 Company: Zoetis
Company: Zoetis
(butorphanol tartrate)
Veterinary Injection and Tablets
NADA 102-990, Approved by FDA
NADA 103-390, Approved by FDA
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
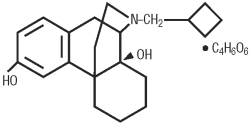
TORBUTROL (butorphanol tartrate) is a narcotic agonist-antagonist analgesic with potent antitussive activity. It is a member of the phenanthrene series. The chemical name is levo-N-cyclobutylmethyl-6, 10 a, ß-dihydroxy-1,2,3,9,10,10a-hexahydro-(4H)-10, 4a- iminoethanophenanthrene tartrate (1:1). It is a white, crystalline, water soluble substance having a molecular weight of 477.56, and its molecular formula is C21H29NO2•C4H6O6.
Chemical Structure
In dogs, the antitussive properties of butorphanol given s.c. were four times more potent than morphine, 10 times more potent than pentazocine (Talwin®-V), and 100 times more potent than codeine. Orally butorphanol is approximately 15 to 20 times more active than either codeine or dextromethorphan.1
Butorphanol given intravenously in large doses (3 mg/kg) to dogs temporarily reduced aortic blood pressure. Aortic pressure returned to baseline control values within 15 to 30 minutes. Changes in cardiac contractile force and cardiac rate were of the same magnitude as the changes in aortic pressure. No appreciable effect on expired carbon dioxide was seen.2 Studies in anesthetized dogs at equianalgetic doses indicate that butorphanol has less potential than morphine for causing airway constriction, hypotension and histamine release.3 In conscious dogs butorphanol produced minimal cardiovascular and respiratory effects.4
The specific site of action of butorphanol is not known. Butorphanol probably exerts analgesic and antitussive effects via the central nervous system (subcortical, possibly the hypothalamus).
TORBUTROL is indicated for the relief of chronic non-productive cough associated with tracheobronchitis, tracheitis, tonsillitis, laryngitis and pharyngitis originating from inflammatory conditions of the upper respiratory tract.
1. The safety of TORBUTROL has not been determined in dogs afflicted with heartworm disease (Dirofilaria immitis).
2. TORBUTROL should not be used in dogs with a history of liver disease.
3. Since TORBUTROL can be effective in totally suppressing cough, it should not be used in conditions of the lower respiratory tract associated with copious mucus production.
FOR USE IN DOGS ONLY.
PRECAUTIONS
1. TORBUTROL has been shown to have potent analgesic activity in rodents; it is undesirable to administer other sedative or analgesic drugs during treatment with TORBUTROL (butorphanol tartrate) as these are likely to produce an additive effect.
2. Reproduction studies, performed in mice and rabbits, revealed no evidence of impaired fertility or harm to the fetus due to butorphanol tartrate. In the rat species the female, on parenteral administration, showed increased nervousness and decreased care for the newborn, resulting in a decreased survival rate of the newborn. This nervousness was seen only in the rat species. There are no well-controlled studies in pregnant bitches but, although there is no well-defined risk, the use of TORBUTROL in pregnant bitches is not recommended.
3. Cough suppression may be accompanied by mild sedation; the degree of sedation is dose related. If sedation is considered undesirable or unnecessary, the dose should be reduced.
4. Toxicity studies indicate that the LD50 in dogs by oral administration is greater than 50 mg/kg. In studies of 4.5 and 13 weeks duration, the following effects were noted in some but not all dogs at 4.6 times the recommended therapeutic dose level BID given parenterally: Decreased activity, weight loss, salivation, elevated SGPT and/or SAP and mild proliferative changes of the bile duct epithelium.
The most frequent adverse reaction reported in 264 dogs treated with oral TORBUTROL was slight sedation in 6 dogs (2.3%). Other less frequent adverse reactions which have been reported include anorexia/nausea and diarrhea (reported incidence less than 1%).
Following the marketing of TORBUTROL Veterinary Injection transient sedation and ataxia have been reported rarely as side effects in dogs.
The usual parenteral dose of TORBUTROL is 0.025 mg of butorphanol base activity per lb of body weight. This is the equivalent of 1/2 mL (0.5 mL) for each 10 lbs of body weight. It should be administered by subcutaneous injection, and repeated at intervals of 6 to 12 hours as required. If necessary, the dose may be increased to a maximum of 0.05 mg/lb or 1 mL/10 lbs body weight. Treatment should not normally be required for longer than seven days.
The usual oral dose of TORBUTROL is 0.25 mg of butorphanol base activity per lb of body weight. This is the equivalent of one 5 mg tablet per 20 lbs of body weight. The dose should be repeated at intervals of 6 to 12 hours as required. If necessary, the dose may be increased to a maximum of one 5 mg tablet for each 10 lbs of body weight. Treatment should not normally be required for longer than seven day
Additional information not available for this medicine.